Medical Research
current Research Studies
1. Clinical outcomes in reconstructive neurosurgery. The members of the Paralysis Center are taking meticulous notes of your findings, surgery, and outcomes in order to determine the efficacy of each procedure in each unique clinical scenario. Because many of the procedures performed here are not common throughout the world, it is important to keep detailed evaluation and outcomes measures so that we can better predict the results of each procedure in each clinical scenario.
2. Anticipating the results of nerve transfers using quantitative neurophysiology. The Paralysis Center uses a sophisticated technique of EMG evaluation that counts the number and shape of motor units within a muscle and sees how these change over time.
We believe that this is the best tool for anticipating the rate and degree of recovery following a nerve injury. We gather this data from each patient that comes through our clinic so that over time we can map out how these findings translated into ultimate strength and functional recovery in each particular muscle group. We think that these findings will change the way other centers manage nerve injuries.
Quantitative Neurophysiology
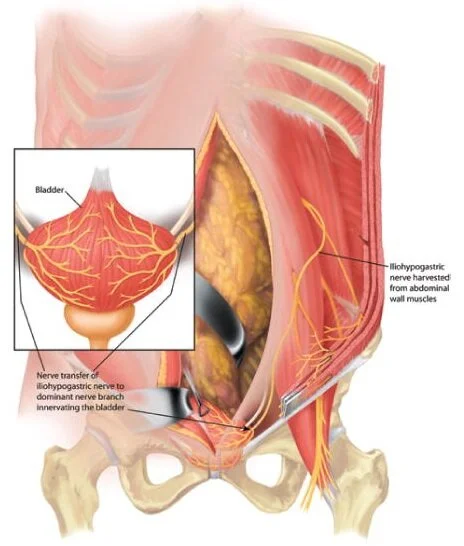
Nerve Transfer for Bladder Function
3. Using nerve transfers to restore bladder control. In conjunction with Dr. Michael Ruggieri and Dr. Mary Barbe at Temple University, Dr. Justin Brown has been studying the potential for restoring the ability to urinate using a nerve transfer surgery. In patients with spinal cord injury, regaining bladder and bowel function is of great importance, in paraplegics it is considered even more important than regaining walking function.
Patents with lower motor neuron lesions resulting in flaccid bladder paralysis cannot use functional electrical stimulation (FES) to induce bladder emptying because the neural connection between the spinal cord and the bladder has been disrupted. These patients require new neural pathways to regain control of bladder function. The goal of these studies is to provide the final burden of proof for human trials of somatic nerve transfer to reinnervate the urinary bladder. We expect to initiate the first human study within the year.
4. Determining the response of cortical motor networks to nerve transfer and the role that rehabilitation plays in supporting cortical plasticity and motor recovery. In conjunction with Dr. Edmund Hollis at Burke Neurological Institute and Weill Cornell Medicine, Dr. Brown is studying the role of innovative therapy programs in achieving optimal function following nerve transfers in patients who have suffered a spinal cord injury. The recovery of hand and arm function is of critical importance for decreasing long-term care costs and increasing quality of life for individuals with tetraplegia due to spinal cord injury (SCI).
A subset of these individuals are candidates for nerve transfer surgery. The use of nerve transfer after SCI is relatively novel and many patients exhibit a remarkable recovery of hand and arm motor function in the months that follow, however others show a much more limited recovery.
The extent of recovery is likely limited, in part, by variability in rehabilitation and the ability of the motor cortex to incorporate the new peripheral circuitry resulting from this surgical procedure. There is a critical need to determine the response of cortical motor networks to nerve transfer and the role that rehabilitation plays in supporting cortical plasticity and motor recovery.
If this need is not met, incomplete recovery from this state-of-the-art surgical intervention will persist and the potential application to a wider patient population will not be realized. This study is a two part investigation, involving both rodent models and human outcomes and is funded by the state of New York. More information about this study can be found here.
5. Transplantation of stem cell-derived spinal motor neurons to facilitate functional restoration following paralyzing injuries. Nerve transfers have evolved into a powerful intervention to restore function following spinal cord injury. However, destruction of the lower motor neurons of the spine and subsequent degeneration of their axons and target muscle remain an impediment to reconstructive strategies. Skeletal muscle requires innervation from these motor neurons to survive and only remain salvageable within a limited time window. Overcoming this degeneration would dramatically augment the functional gains realized by long distance nerve repairs in the distal upper extremity and open opportunities not previously explored for devastating injuries affecting the lower body.
In collaboration with world-renowned stem cell expert, Dr. Kevin Eggan, at Harvard University we are investigating an innovative approach of transplanting stem cell-derived spinal motor neurons into the distal nerve to innervate and sustain muscle integrity while the native, regenerating axons make the long distance journey to the target muscle. These transplanted cells may also serve to directly drive muscle contraction upon electrical or optogenetic stimulation from an external source such as a brain computer interface.
Induced pluripotent stem cells (IPSCs) are used in this study. These cells can be directed to become any cell in the human body. Unlike, embryonic stem cells, there is no destruction of human embryos, as they are derived from adult tissue, such as a skin sample. Furthermore, adult-derivation enables the potential for autologous cell transplantation, obviating the need for immunosuppression.